Laser-Induced Fluorescence Primer II: Interpreting Waveforms
Randy St. Germain, President
Ultraviolet (UV) laser-induced fluorescence (LIF) screening tools cause fluorescence in light non-aqueous phase liquids (LNAPLs). Total fluorescence readings estimate the quantity of LNAPL present, but LIF can also capture fluorescence "waveforms" to qualitatively evaluate LNAPL type.
[This article focuses on UV LIF’s application to petroleum fuels and oils, but NOT creosotes and coal tar. These require the Tar-specific Green Optical Screening Tool or TarGOST. TarGOST and the problem inherent with the fluorescence of heavier hydrocarbons will be discussed in a forthcoming LIF Line]
BACKGROUND:
Optical screening tools (OSTs) “flash” a sample with intense light for a few nanoseconds, then analyze the fluorescence that continues to be emitted long after the flash has stopped. This fluorescence “lifetime” varies, from well under a nanosecond to tens or even hundreds of nanoseconds.
OSTs have this time-resolved LIF capability built in so that they can simultaneously record the “color” (spectral nature) and lifetime (temporal nature) of the fluorescence. OST LIF systems can tentatively identify the type of petroleum, discern false positives, and detect weathering of certain LNAPLs with lifetimes playing a major role.
MULTI-WAVELENGTH WAVEFORMS:
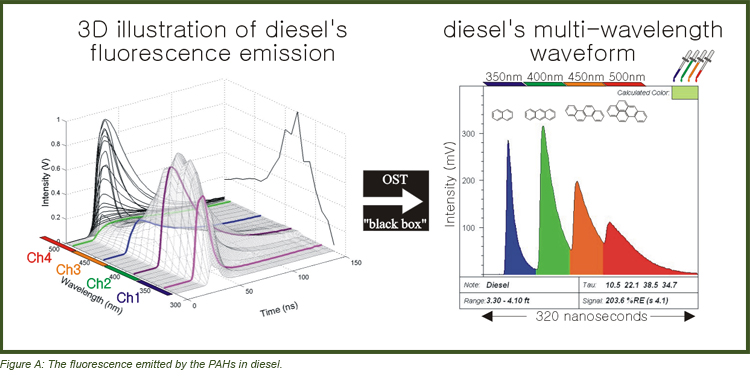
The left side of Figure A illustrates the combined spectral/temporal nature of the fluorescence emitted by the polycyclic aromatic hydrocarbons (PAHs) in diesel, and the right side shows the 2D “shorthand” style waveform characteristic of OST data.
Key characteristics of waveforms include:
Intensity (Amount of Fluorescence)
The y-axis represents the intensity or “brightness” of the fluorescence. It is a voltage.
• Intensity generally increases with increasing fuel/oil pore saturation.
• The relative fluorescence intensity is dependent on the composition of the fuel.
o Some fuels (diesel and crude) fluoresce more intensely than others (gasoline).
o Low viscosity, high solvent content fuels/oils dominated by smaller 2-3 ring PAHs usually fluoresce better (higher voltages) than large PAHs containing fuels, oils, greases or sludge.
The Four Fluorescence Channels (“Peaks”)
The x-axis of Figure A represents 320 nanoseconds of time during which the four wavelength ranges of fluorescence arrive at the detector at sequentially delayed times.
• The four peaks or channels represent 350 nm (blue), 400 nm (green), 450 nm (orange), and 500 nm (red). Each peak is 40 nm wide.
• It is important to note that the blue, green, orange, and red aren’t the true colors of those wavelengths – just colors chosen to represent the four peaks.
• The relative intensities between the four channels is used to fill the LIF log with blended color to visualize fluorescence trends “at a glance”. [note the fill-color boxes in upper right corner of the example waveforms to follow]
• OST systems are “calibrated” by applying the Reference Emitter (RE) fluid to the window and recording RE’s response. The RE is a standard fluorescing NAPL supplied by Dakota to all OST service providers for the last 15 years. The purpose of the RE is to:
1. Normalize the data for push-to-push fluctuations in optical throughput.
2. System check to make sure all optics are intact and operating normally.
• All downhole data is normalized to the RE (as percentage) so that data is consistent across all OSTs across the globe, regardless of who is operating them.
• The factory RE is displayed on the oscilloscope along with the latest RE, assisting the operator in assuring that the OST data they generate “matches” all other OSTs.
• The area under the curve of all four channels is summed and divided by the RE waveforms’ areas to generate the normalized total fluorescence “Signal” of an LIF log.
• One PAH will usually occupy more than one channel since PAHs fluoresce broadly (naphthalene is a notable exception, fluorescing almost exclusively in the blue channel).
• In general, the shorter wavelength channels (blue/green) are occupied by 2- and 3-ring PAHs, the middle (green/orange) by 3- and 4-ring PAHs, and the rightmost (orange/red) by 4-ring and larger PAHs [Figure A].
• The voltage or height of the waveform generally scales with NAPL saturation for any single NAPL on a single soil type.
Lifetimes (Decaying Fluorescence)
The x-axis of waveforms represents the extremely short time period necessary to capture the pulse of PAH fluorescence. The lifetime is the average time the laser-induced PAH population stays in the excited state prior to fluorescing.
• PAHs fluoresce from a few to hundreds of nanoseconds after excitation.
• In the characteristic diesel example, the blue channel has a shorter lifetime than the green channel (see how the intensity falls back toward baseline more quickly on the right side of the 350 nm peak).
• The lifetimes of some channels “bleed” into subsequent channels (green into orange, for instance) and influence the log’s fill colors.
• Short lifetimes often indicate an energy transfer from smaller excited-state PAHs to surrounding, larger PAHs or the matrix. This leads to quenching (reduction) of fluorescence and red-shifting of the emission toward the right (longer wavelength).
• Long lifetimes often indicate oxygen starvation and/or a solvent-rich fluorescent friendly environment. Natural gas condensates can have unusually long lifetimes.
EXAMPLE WAVEFORMS:
PAHs:
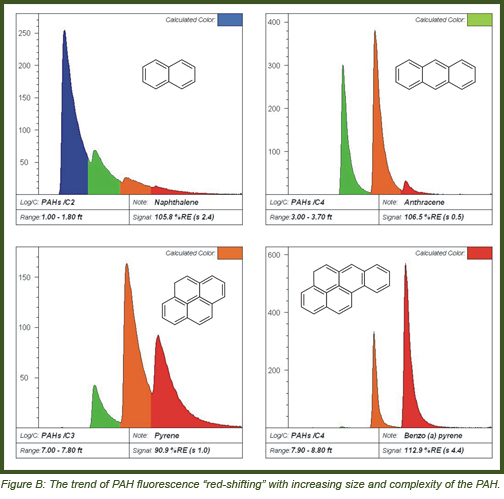
PAH waveforms show the general trend of emission with size/complexity of PAHs (Figure B).
• The upper-left is Naphthalene, fluorescing almost entirely in the blue channel.
• The upper-right is Anthracene, the simple addition of one more benzene ring onto a naphthalene shifted the fluorescence by about 75-100 nm (about 1.5-2 “channels”).
• The lower-left is Pyrene, which is one ring larger but more “compact” so the red-shifting is moderate comparatively.
• The lower-right is Benzo (a) pyrene, emitting almost entirely in the red channel.
Fuels/Oils:
Fuels/oils contain complex chemical composition, which results in broad waveforms. The “EPA 16” PAHs only account for approximately 1 percent of the PAHs in crude oil, so there are usually a wide variety of PAHs fluorescing. From “blue to red” some common fuel/oil waveforms, shown in Figure C, include the following:
• Jet/Kerosene [Row 1]:
o Naphthalene and Jet/Kerosene waveforms are similar because those fuels’ PAH content are dominated by the naphthalenes.
• Gasoline [Row 2]:
o Intact (unweathered) gasoline has the typical shape shown and will fluoresce well enough to be delineated with LIF, even though gasoline contains low concentrations of 2- to 3-ring PAHs relative to the benzene, toluene, ethylebenzene and xylenes (BTEX) and aliphatics.
• Diesel [Row 3]:
o Diesel has a short lifetime blue (2-ring PAH) peak that is about the same height as the longer lifetime green peak. Evidence of weathering in diesels is rare.
• Oils [Row 4]:
o Oils such as crude, lubricating, and cutting types fluoresce well and are usually found slightly right of center (red-shifted) and have medium to long lifetimes.

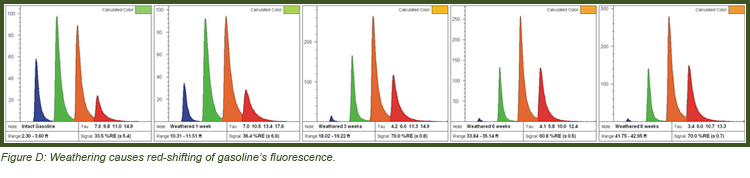
Weathering:
Weathering typically removes the most soluble, volatile, and readily metabolized PAHs first. Large PAHs remain behind, which causes a red-shift in the fluorescence waveform. Simultaneously there is a loss of solvent aliphatics, which often shortens the lifetime so a weathered light fuel will start to look like oil or even tar. Figure D demonstrates weathering of gasoline in a lab experiment, resulting in red-shifting that can make weathered gasoline display an “oil” waveform. When jet/kerosene weather, they simply “disappear” (become non-fluorescent) because there are too few larger PAHs to fluoresce once the naphthalenes are gone. It is important to appreciate that NAPL (especially gasolines) can vary in their appearance even prior to spilling. The degree of red-shifting is relative to the starting product and site conditions. For instance, one site’s intact (unweathered) gasoline waveform might very well be identical to different site’s moderately weathered gasoline.
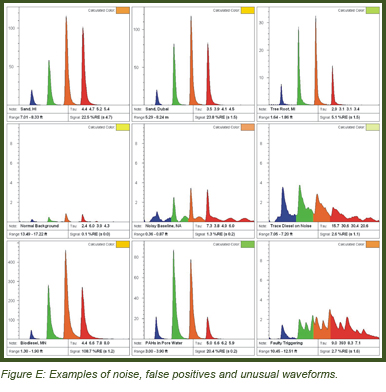
False Positives and Oddities:
Areas with little to no contamination may fluoresce, which generates “false positives.” Figure E contains a variety of noise, false-positives, or highly unusual waveforms. Be prepared to obtain samples in order to figure out what materials are causing any odd fluorescence waveforms.